Harnessing Ammonia's Nitrogen Component for Food Production
At the beginning of the 20th Century, it was predicted that naturally occurring deposits of nitrogen could not satisfy future demands, with the risk of a shortage and negative impact on food production ahead. The Haber-Bosch process solved this challenge by fixing atmospheric nitrogen with hydrogen to produce anhydrous ammonia (NH3) - the building block for all nitrogen products.
Along with advancements in seed technology and farming practices, the growing use of nitrogen fertilizer and other nutrients dramatically increased food production in the second half of the 1900s and lifted countless people out of hunger. At the same time, because fertilizer increases yield, it allows more food to be grown on fewer acres. This reduces the amount of land cleared for agriculture, preserving carbon sequestering forests and important wildlife ecosystems.
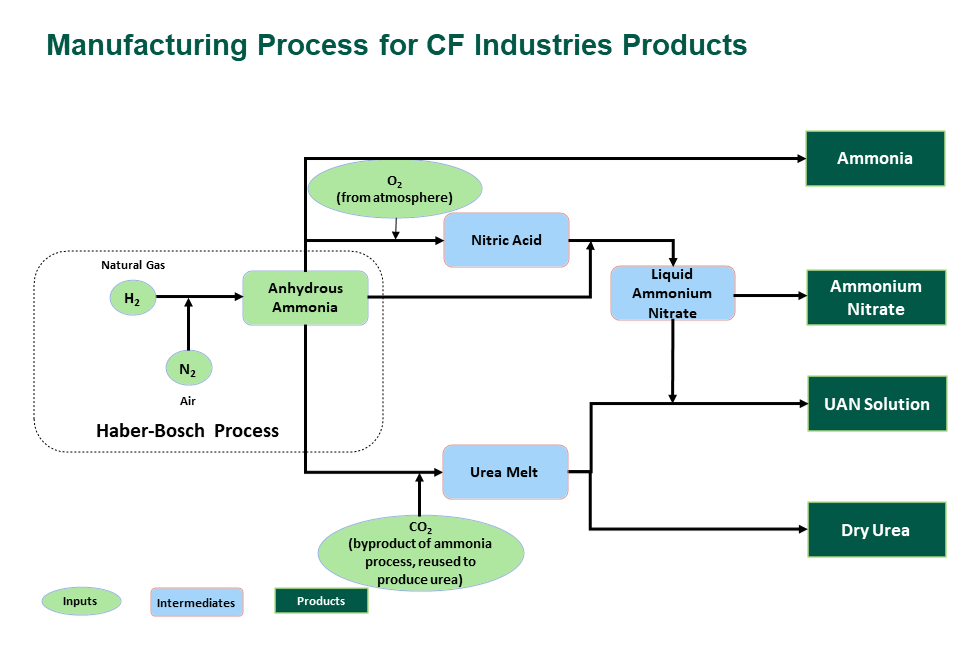
Fertilizer Products
CF Industries produces four nitrogen-based fertilizer products.
